Cancer medicinal chemistry
Identification of small molecule agents that can induce selective differentiation of cancer stem like cells (CSLCs) to more benign states leading to anti-tumour activity in vivo.
With Professor Thomas Milne and Professor Paresh Vyas (Weatherall Institute of Molecular Medicine; WIMM, University of Oxford), Professor Tariq Enver (University College London), Professor Petter Woll and Professor Sten Eirik Jacobsen (Karolinska Institute, Sweden)
Acute Myeloid Leukaemia (AML)

Acute Myeloid Leukaemia (AML) is a type of blood cancer characterised by the rapid growth of abnormal immature blood cells. In a healthy bone marrow, blood stem cells will differentiate into several types of mature blood cells: white blood cells, red blood cells and platelets. In AML, this differentiation process is disrupted, which leads to the formation of immature white blood cells that accumulate in the bone marrow and interfere with the formation of functional blood cells of all types.
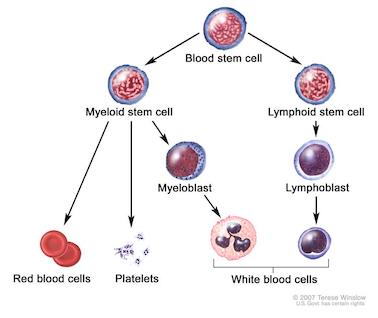
Blood cell development (Reference: National Cancer Institute)
Despite scientific advances, clinical outcomes for patients with AML remain poor and the majority of patients relapse after treatment. While most current treatments aim to kill the malignant AML cells, our approach is to instead overcome the differentiation block using small molecules. We have so far developed a screening method and identified some promising small molecules capable of differentiating multiple AML cell types in vitro. We have progressed our most promising compounds into an in vivo model of AML and shown they significantly reduced tumour growth.
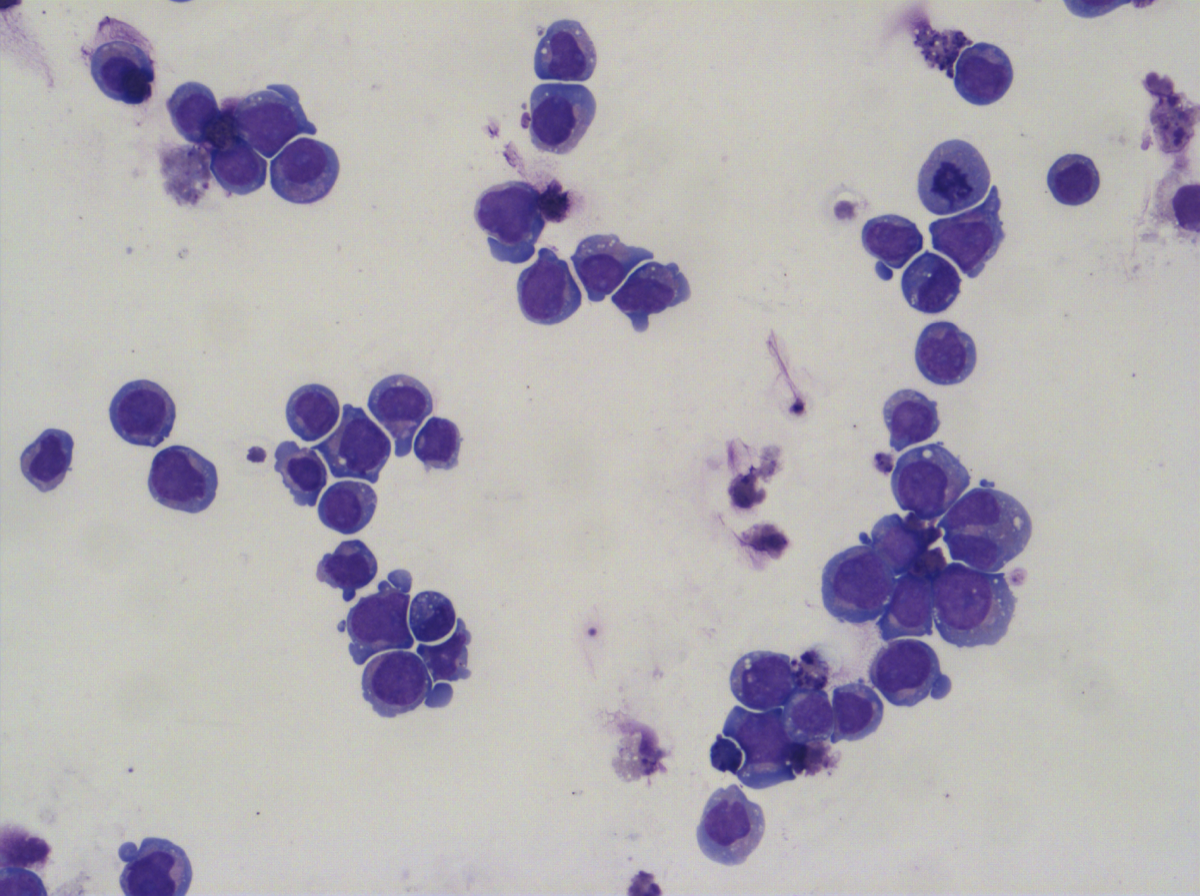
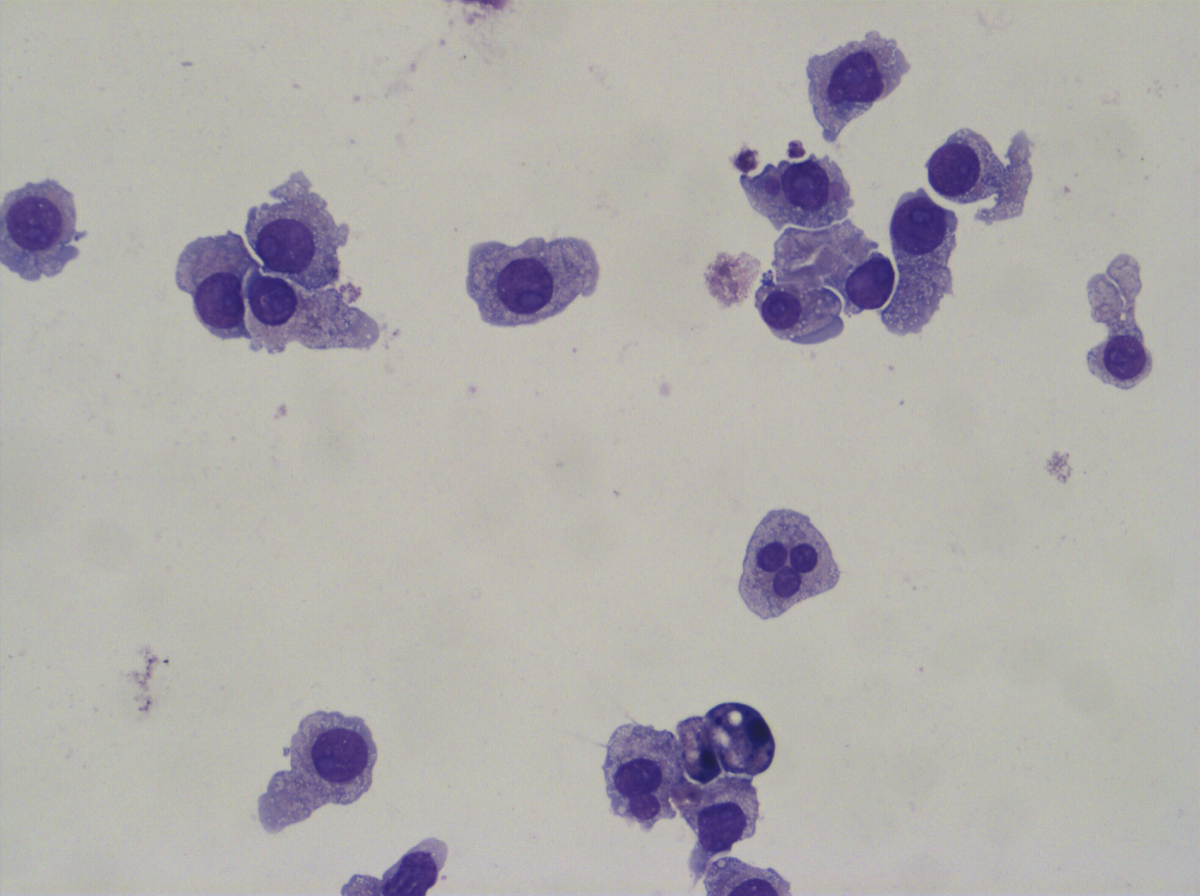
a) Malignant AML cells; b) differentiated AML cells
Serine/Threonine kinase inhibitors as anti-tumour agents
With Professor Stefan Knapp (Nuffield Department of Medicine, University of Oxford; Structural Genomics Consortium).

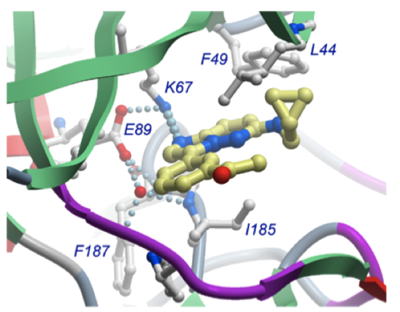
The PIM family (PIM1-3) of serine/threonine kinases phosphorylate a number of proteins with important roles in cell cycle progression, transcriptional activation and signal transduction pathways. PIM is overexpressed in several tumour types, with evidence emerging that it is a key driver of tumourigenesis. Inhibition of PIM kinases represents an exciting potential strategy for the treatment of various cancers and we are working to develop selective small molecule PIM inhibitors as targeted anti-cancer agents.
Highly selective inhibitors have been generated which show good exposure and excellent potency in cellular assays against both prostate cancer and leukemia cell lines. Analysis of cell lysates demonstrates a dose-dependent reduction in levels of biomarkers for PIM activity (e.g. p4EBP1, c-Myc).
Chemical probes for tumour biomarker detection and inhibition
With Professor Edith Sim (Faculty of Science, Engineering and Computing, Kingston University London)

Human arylamine N-acetyltransferase 1 (HUMAN(NAT1)) is one of the ten most highly over-expressed genes in ER+ breast tumours, and has been proposed as a candidate diagnostic biomarker and drug target in ER+ breast cancer. In collaboration with Professor Edith Sim (Faculty of Science, Engineering and Computing, Kingston University London), we discovered a potent and specific inhibitor of HUMAN(NAT1), which we found to undergo a distinctive colour change (red to blue) upon binding to HUMAN(NAT1) or its murine homologue but not in the presence of any other enzyme tested.
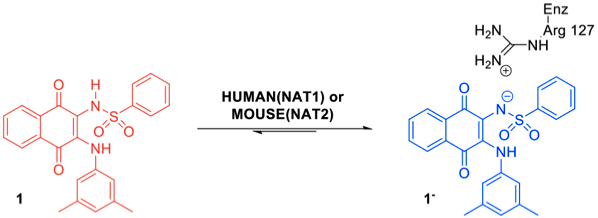
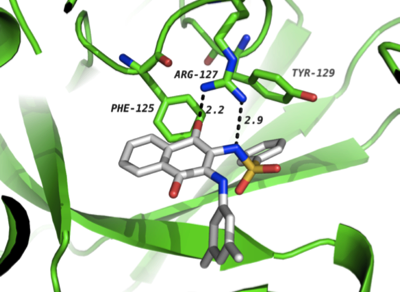
A combination of chemical, biochemical and computational studies revealed that this colour change was due to an unprecedented selective binding of its conjugate base to HUMAN(NAT1), driven by an ionic interaction with the Arg127 residue. We have subsequently used these probes to uncover the functional role of NATs and to develop of fluorimetric quantitative detection method to sense HUMAN(NAT1) in tumour cells. We have also published work on the development of inhibitors of other anticancer targets.
References
(1) Russell, A. J.; Westwood, I. M.; Crawford, M. C.; Robinson, J.; Kawamura, A.; Redfield, C.; Lowe, E. D.; Laurieri, N.; Davies, S. G. and Sim, E. Selective small molecule inhibitors of the potential breast cancer marker, human arylamine N-acetyltransferase 1, and its murine homologue, mouse arylamine N-acetyltransferase 2. Bioorg. Med. Chem. 2009, 17, 905–918.
(2) Laurieri, N.; Crawford, M. H. J.; Kawamura, A.; Westwood, I. M.; Robinson, J.; Fletcher, A. M.; Davies, S. G.; Sim, E. and Russell, A. J. Small Molecule Colorimetric Probes for Specific Detection of Human Arylamine N-Acetyltransferase 1, a Potential Breast Cancer Biomarker. J. Am. Chem. Soc. 2010, 132, 3238–3239.
(3) Laurieri, N.; Egleton, J.E.; Varney, A.; Thinnes, C. C.; Quevedo, C. E.; Seden, P.T.; Thompson, S.; Rodrigues-Lima, F.; Dairou, J.; Dupret, J.-M.; Russell, A. J.; Sim, E. A Novel Color Change Mechanism for Breast Cancer Biomarker Detection: Naphthoquinones as Specific Ligands of Human Arylamine N-Acetyltransferase 1. PLoS One, 2013, 8, e70600.
(4) Egleton, J. E.; Thinnes, C. C.; Seden, P. T.; Laurieri, N.; Lee, S. P.; Hadavizadeh, K. S.; Measures, A. R.; Jones, A. M.; Thompson, S.; Varney, A.; Wynne, G. M.; Ryan, A.; Sim, E.; Russell, A. J. Structure-activity relationships and colorimetric properties of specific probes for the putative cancer biomarker human arylamine N-acetyltransferase 1. Bioorg. Med. Chem. 2014, 22, 3030–3054.