Publications
Key publications
1. Wilkinson. I.V.L., Perkins, K.J., Dugdale, H., Moir, L., Vuorinen, A., Chatzopoulou, M., Squire, S.E., Monecke, S., Lomow, A., Geese, M., Charles, P.D., Burch, P., Tinsley, J.M., Wynne, G.M., Davies, S.G., Wilson, F.X., Rastinejad, F., Mohammed, S., Davies, K.E., Russell, A.J. Chemical Proteomics and Phenotypic Profiling Identifies the Aryl Hydrocarbon Receptor as a Molecular Target of the Utrophin Modulator Ezutromid. Angewandte Chemie, 2019. DOI: https://doi.org/10.1002/anie.201912392
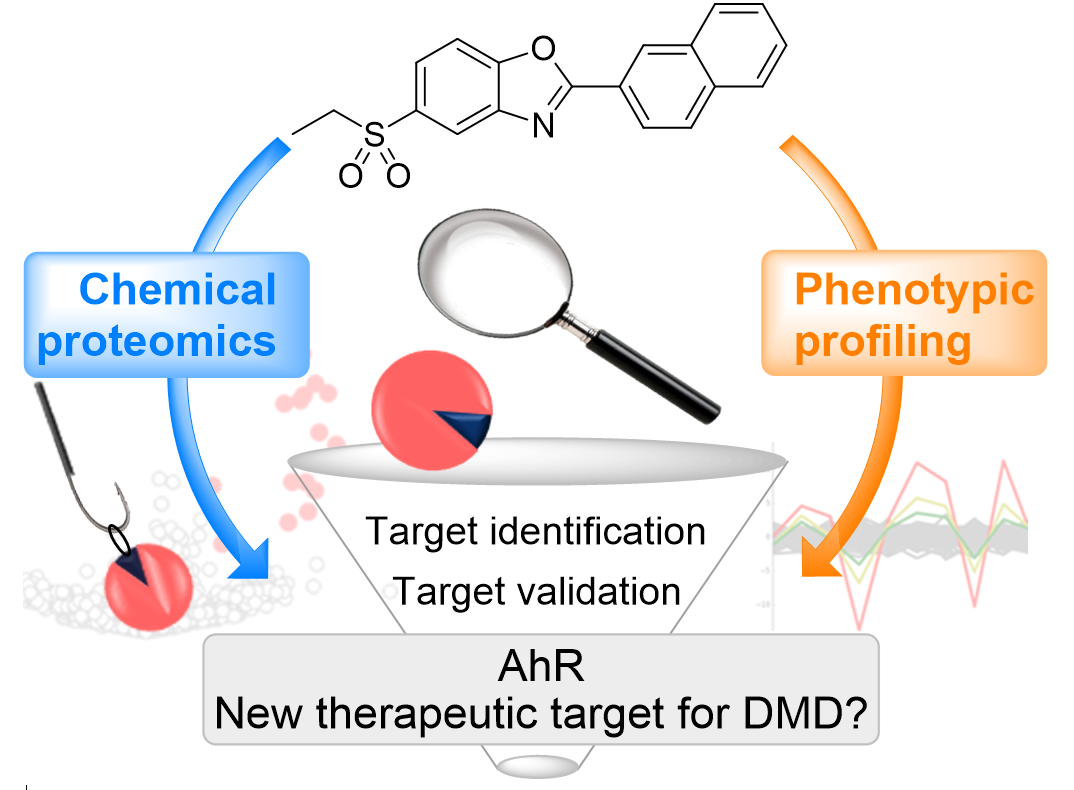
This paper describes the first identification and validation of a disease-modifying target for Duchenne muscular dystrophy (DMD), through target deconvolution of the clinical compound ezutromid. This is a real breakthrough in the field as there is currently no cure for DMD and this work enables the first target‐based drug discovery program in DMD.
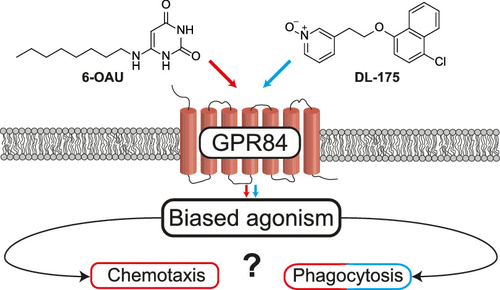
2. Lucy, D., Purvis, G.S.D,. Zeboudj, L., Chatzopoulou, M., Recio, C., Bataille, C.J.R., Wynne, G.M., Greaves, D.R., and Russell, A.J. A Biased Agonist at Immunometabolic Receptor GPR84 Causes Distinct Functional Effects in Macrophages. ACS Chem. Biol. 2019, 14, 2055-2064. DOI: https://doi.org/10.1021/acschembio.9b00533
In collaboration with David Greaves' lab (Sir William Dunn School of Pathology) this study in ACS Chemical Biology reveals how biased signalling at the GPR84 receptor can result in differing regulation of immune cell chemotaxis.
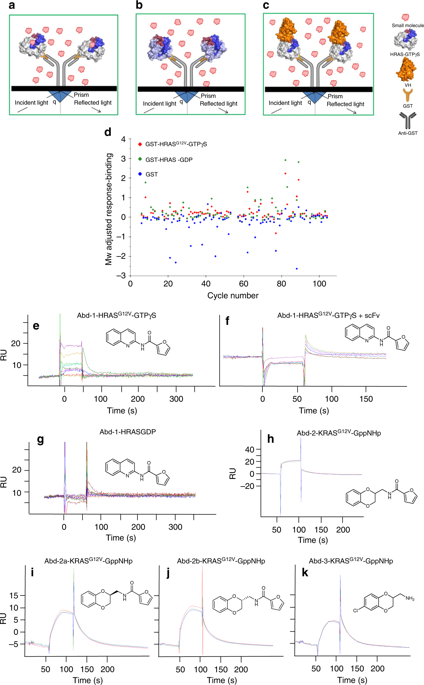
3. Quevedo, C.E., Cruz-Migoni, A., Bery, N., Miller, A.,Tanaka, T., Petch, D., Bataille, C.J.R., Lee, L.Y.W., Fallon, P.S., Tulmin, H., Ehebauer, M.T., Fernandez-Fuentes, N., Russell, A.J., Carr, S.B., Phillips, S.E.V., & Rabbitts, T.H. Small molecule inhibitors of RAS-effector protein interactions derived using an intracellular antibody fragment, Nature Communications, 2018, 9. DOI: 10.1038/s41467-018-05707-2
4. Kerr, A.G.; Tam, L.C.S.; Hale, A.B.; Cioroch, M.; Douglas, G.; Agkatsev, S.; Hibbitt, O.; Mason, J.; Holt-Martyn, J.; Bataille, C.J.R.; Wynne, G.M.; Channon, K.M.; Russell, A.J. and Wade-Martins, R. A genomic DNA reporter screen identifies squalene synthase inhibitors which act cooperatively with statins to upregulate the low-density lipoprotein receptor. Journal of Pharmacology and Experimental Therapeutics, 2017, 361 (3), 417-428. DOI: https://doi.org/10.1124/jpet.116.239574
Joint programme of research with the Wade-Martins group. The discovery of a novel series of small molecules as upregulators of the low-lipodensity receptor (LDLR) in vitro and in vivo, their molecular mechanism of action and their cooperative effects with statins elucidated in vitro. They thus may serve as a means to treat diseases such as familial hypercholesterolaemia which cannot always be adequately controlled with statins alone.
5. Partridge, FA.; Murphy, EA.; Willis, NJ.; Bataille, CJR.; Forman, R.; Heyer-Chauhan, N.; Marinič, B.; Sowood, DJC.; Wynne, GM.; Else, KJ.; Russell, AJ.; Sattelle, DB. Dihydrobenz[e][1,4]oxazepin-2(3H)-ones, a new anthelmintic chemotype immobilising whipworm and reducing infectivity in vivo. PLoS Negl Trop Dis, 2017, 11(2): e0005359. DOI:10.1371/ journal.pntd.0005359.
Joint programme of research with Else and Sattelle in a multiway collaborative open innovation drug discovery programme. The discovery of a novel series of small molecules as anthelmintics with activity both in vitro and in vivo against whipworm, an inadequately controlled disease prevalent in developing countries.
6. Gianella-Borradori, M.; Christou, I.; Bataille, C. J. R.; Cross, R. L.; Wynne, G. M.; Greaves, D. R.; Russell, A. J. Ligand-based virtual screening identifies a family of selective cannabinoid receptor 2 agonists. Bioorg. Med. Chem. 2015, 23, 241-263. DOI: https://doi.org/10.1016/j.bmc.2014.11.002
Joint programme of research with Greaves. The discovery of a novel series of small molecules as highly selective cannabinoid receptor 2 (CB2R) agonists with downstream application as endocannabinoid modulating anti-inflammatory agents. The highly selective CB2R agonists have been subsequently used to demonstrate the polypharmacology of previously described CB2R agonists (see Taylor et al., Scientific Rep. 2015).
7. Davies, S. G.; Kennewell, P. D.; Russell, A. J.; Seden, P. T.; Westwood, R.; Wynne, G. M. Stemistry: The Control of Stem Cells in Situ Using Chemistry. J. Med. Chem. 2015, 58, 2863-2894. DOI: 10.1021/jm500838d
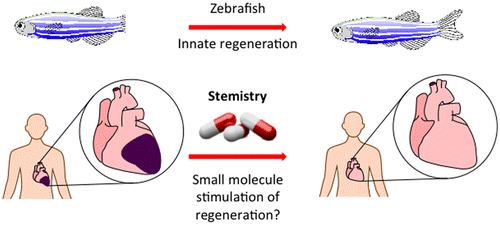
A field-defining invited and peer-reviewed perspective article introducing the concept of “Stemistry”: in situ stem cell chemistry, a term now adopted by the field. Provides a critical overview of the work of our group and others developing drugs for regenerative medicine by targeting and augmenting endogenous repair pathways directly in vivo.
8. Laurieri, N.; Crawford, M. H. J.; Kawamura, A.; Westwood, I. M.; Robinson, J.; Fletcher, A. M.; Davies, S. G.; Sim, E. and Russell, A. J. Small Molecule Colorimetric Probes for Specific Detection of Human Arylamine N-Acetyltransferase 1, a Potential Breast Cancer Biomarker. J. Am. Chem. Soc. 2010, 132, 3238–3239. DOI: 10.1021/ja909165u
The paper describes a novel and simple mechanism through which a small molecule ligand’s optical absorption properties could be used to specifically detect binding to a native protein. The molecules were subsequently developed into tools to quantify this specific protein within tumour cells. Highlighted in Anslyn, E. Supramolecular and Chemical Cascade Approaches to Molecular Sensing, J. Am. Chem. Soc. 2010, 132, 15833–15835; JACS Select #10 “Supramolecular and Chemical Cascade Approaches to Molecular Sensing”.
9. Soncin, F.; Mohamet, L.; Eckardt, D.; Ritson, S.; Eastham, A. M.; Spencer, H.; Bobola, N.; Russell, A. J.; Davies, S. G.; Kemler, R.; Merry, C. L. R. and Ward, C. M. Abrogation of E-cadherin mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells, 2009, 27, 2069–2080. DOI: 10.1002/stem.134
Dogma-challenging paper that demonstrated by genetic and chemical means that embryonic stem cells (ESCs) could be cultured in a contact-independent manner while maintaining viability and pluripotency. Our research implemented the chemical-induced cell-cell segregation aspect – specifically targeting E-cadherin. This work has been instrumental in enabling scale-up culture of mouse ESCs and, importantly, subsequent translation to human cells.
Book chapters
Davies, S.G., Kennewell, P.D., Russell, A.J., Silpa, L., Westwood, R., and Wynne, G.M. Regenerative Medicine. Comprehensive Medicinal Chemistry III, Chackalamannil, S., Rotella, D., and Ward, S.E. (Ed.). Elsevier Ltd, 2017, ISBN: 978-0-12-803201-5.
Russell, A. J. and Wynne, G. M.
Drug Discovery Approaches for Rare Neuromuscular Diseases. Orphan Drugs and Rare Diseases, Palmer, M. and Pryde, D. (Ed.). Royal Society of Chemistry, 2014, ISBN: 978-1-84973-806-4.
Davies, S. G. and Russell, A. J.
Chemical Biology of Stem Cell Modulation
New Frontiers in Chemical Biology, Bunnage, M. E. (Ed.). Royal Society of Chemistry, 2011, ISBN: 184973125X.
All publications
2020
99 Babbs, A., Berg, A., Chatzopoulou, M., Davies, K.E., Davies, S.G., Edwards, B., Elsey, D.J., Emer, E., Figuccia, A.L.A., Fletcher, A.M., Guiraud, S., Harriman, S., Moir, L., Robinson, N., Rowley, J.A., Russell, A.J., Squire, S.E., Thomson, J.E., and Wynne, G.M. Synthesis of SMT022357 enantiomers and in vivo evaluation in a Duchenne muscular dystrophy mouse model. Tetrahedron, 76 (2), 2020, 130819. DOI: https://doi.org/10.1016/j.tet.2019.130819
98 Wilkinson, I.V.L., Reynolds, J.K., Galana, S.R.G., Vuorinena, A., Sills, A.J., Piresa, E., Wynne, G.M., Wilson, F.X., Russell, A.J. Characterisation of utrophin modulator SMT C1100 as a non-competitive inhibitor of firefly luciferase. Bioorganic Chemistry, 94, 2020, 103395. DOI: https://doi.org/10.1016/j.bioorg.2019.103395
2019
97 Wilkinson. I.V.L., Perkins, K.J., Dugdale, H., Moir, L., Vuorinen, A., Chatzopoulou, M., Squire, S.E., Monecke, S., Lomow, A., Geese, M., Charles, P.D., Burch, P., Tinsley, J.M., Wynne, G.M., Davies, S.G., Wilson, F.X., Rastinejad, F., Mohammed, S., Davies, K.E., Russell, A.J. Chemical Proteomics and Phenotypic Profiling Identifies the Aryl Hydrocarbon Receptor as a Molecular Target of the Utrophin Modulator Ezutromid. Angewandte Chemie, 2019. DOI: https://doi.org/10.1002/anie.201912392
96 Chatzopoulou M., Claridge T.D.W., Davies K.E., Davies S.G., Elsey D., Emer E., Fletcher A.M., Harriman S., Robinson N., Rowley J., Russell A.J., Tinsley J., Weaver R., Wilkinson I., Willis N., Wilson F., Wynne G.M.Isolation, structural identification, synthesis, and pharmacological profiling of 1,2-trans-dihydro-1,2-diol metabolites of the utrophin modulator ezutromid. Journal of Medicinal Chemistry. 2019. DOI: https://doi.org/10.1021/acs.jmedchem.9b01547
95 Lucy, D., Purvis, G.S.D,. Zeboudj, L., Chatzopoulou, M., Recio, C., Bataille, C.J.R., Wynne, G.M., Greaves, D.R., and Russell, A.J. A Biased Agonist at Immunometabolic Receptor GPR84 Causes Distinct Functional Effects in Macrophages. ACS Chem. Biol. 2019, 14, 2055-2064. DOI: https://doi.org/10.1021/acschembio.9b00533
94 Cruz-Migoni A; Canning P; Quevedo CE; Bataille CJR; Bery N; Miller A; Russell AJ; Phillips SEV; Carr SB; Rabbitts TH. Structure-based development of new RAS-effector inhibitors from a combination of active and inactive RAS-binding compounds. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116, 2545-2550. DOI: https://doi.org/10.1073/pnas.1811360116
2018
93 Partridge, F.A., Forman, R. Willis, N.J., Bataille, C.J.R., Murphy, E.A., Brown, A.E., Heyer-Chauhan, N., Marinič, B., Sowood, D.J.C., Wynne, G.M., Else, K.J., Russell, A.J., and Sattelle, D.B. 2,4-Diaminothieno[3,2-d]pyrimidines, a new class of anthelmintic with activity against adult and egg stages of whipworm. PLoS Neglected Tropical Diseases, 2018, DOI: https://doi.org/10.1371/journal.pntd.0006487
92 Recio, C., Lucy, D., Purvis, G.S.D., Iveson, P., Zeboudj, L., Iqbal, A.J., Lin, D., O'Callaghan, C., Davison, L., Griesbach, E., Russell, A.J., Wynne, G.M., Dib, L., Monaco, C., and Greaves, D.R. Activation of the Immune-Metabolic Receptor GPR84 Enhances Inflammation and Phagocytosis in Macrophages. Front. Immunol. 2018, 9:1419. DOI: 10.3389/fimmu.2018.01419
91 Partridge, F. A.; Brown, A. E.; Buckingham, S. D.; Willis, N. J.; Wynne, G. W.; Forman, R.; Else, K. J.; Morrison, A.; Matthews, J. B.; Russell, A. J.; Lomas, D. J.; Sattelle, D. B. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. Int. J. Parasitol.: Drugs and Drug Resistance, 2018, 8, 8-21. DOI: 10.1016/j.ijpddr.2017.11.004
90 Bery, N., Cruz-Migoni, A., Bataille, C.J.R., Quevedo, C.E., Tulmin, H., Miller, A., Russell, A.J., Phillips, S.E.V., Carr, S.B., and Rabbitts, T.H. BRET-based RAS biosensors that show a novel small molecule is an inhibitor of RAS-effector protein-protein interactions. eLife, 2018;7:e37122 DOI: 10.7554/eLife.37122
89 Stefaniak, J., Lewis, A.M., Conole, D., Galan, S.R.G., Bataille, C.J.R., Wynne, G.M., Castaldi, M.P., Lundbäck, T., Russell, A.J., and Huber, K.V.M. Chemical Instability and Promiscuity of Arylmethylidenepyrazolinone-Based MDMX Inhibitors. ACS Chem. Biol. 2018, DOI: 10.1021/acschembio.8b00665
88 Venturi, E., Lindsay, C., Lotteau, S., Yang, Z., Steer, E., Witschas, K., Wilson, A. D., Wickens, J. R., Russell, A. J., Steele, D., Calaghan, S., and Sitsapesan, R. Simvastatin activates single skeletal RyR1 channels but exerts more complex regulation of the cardiac RyR2 isoform. British Journal of Pharmacology, 2018. DOI: 10.1111/bph.14136.
87 Quevedo, C.E., Cruz-Migoni, A., Bery, N., Miller, A.,Tanaka, T., Petch, D., Bataille, C.J.R., Lee, L.Y.W., Fallon, P.S., Tulmin, H., Ehebauer, M.T., Fernandez-Fuentes, N., Russell, A.J., Carr, S.B., Phillips, S.E.V., & Rabbitts, T.H. Small molecule inhibitors of RAS-effector protein interactions derived using an intracellular antibody fragment, Nature Communications, 2018, 9. DOI: 10.1038/s41467-018-05707-2
86 Chatzopoulou, M., Martínez, R.F., Willis, N.J., Claridge, T.D.W., Wilson, F.X., Wynne, G.W., Davies, S.G. and Russell, A.J. The Dimroth rearrangement as a probable cause for structural misassignments in imidazo[1,2-a]pyrimidines: A 15N-labelling study and an easy method for the determination of regiochemistry. Tetrahedron, 2018, 74(38), 5280-5288. DOI: https://doi.org/10.1016/j.tet.2018.06.033
85 Recio, C., Lucy, D., Iveson, P., Iqbal, A.J., Valaris, S., Wynne, G.M., Russell, A.J., Choudhury, R., O'Callaghan, C., Monaco, C., and Greaves, D.R. The Role of Metabolite-sensing G Protein-Coupled Receptors in inflammation and metabolic disease. Antioxidants & Redox Signaling 2018, DOI: 10.1089/ars.2017.7168.
2017
84 Kerr, A.G.; Tam, L.C.S.; Hale, A.B.; Cioroch, M.; Douglas, G.; Agkatsev, S.; Hibbitt, O.; Mason, J.; Holt-Martyn, J.; Bataille, C.J.R.; Wynne, G.M.; Channon, K.M.; Russell, A.J. and Wade-Martins, R. A genomic DNA reporter screen identifies squalene synthase inhibitors which act cooperatively with statins to upregulate the low-density lipoprotein receptor. Journal of Pharmacology and Experimental Therapeutics, 2017, 361 (3), 417-428. DOI: https://doi.org/10.1124/jpet.116.239574
83 Partridge, FA.; Murphy, EA.; Willis, NJ.; Bataille, CJR.; Forman, R.; Heyer-Chauhan, N.; Marinič, B.; Sowood, DJC.; Wynne, GM.; Else, KJ.; Russell, AJ.; Sattelle, DB. Dihydrobenz[e][1,4]oxazepin-2(3H)-ones, a new anthelmintic chemotype immobilising whipworm and reducing infectivity in vivo. PLoS Negl Trop Dis, 2017, 11(2): e0005359. DOI:10.1371/ journal.pntd.0005359.
82 Brambilla, M, Brennan, M.B., Csatayová, K., Davies, S.G., Fletcher, A.M., Kennett, A.M.R., Lee, J.A., Roberts, P.M., Russell, A.J., Thomson, J.E. Probing Competitive and Co-operative Hydroxyl and Ammonium Hydrogen-Bonding Directed Epoxidations. J Org Chem, 2017, 82, 10297-10309. DOI: 10.1021/acs.joc.7b01774.
81 Tempone, A.G.; Ferreira, D.D.; Lima L.M.; Silva, T.A.C.; Borborema, S.E.T.; Reima, J.Q.; Galuppo, M.K.; Guerra, J.M.; Russell, A.J.; Wynne, G.M.; Lai, R.Y.L.; Cadelis, M.M.; Copp, B.R. Efficacy of a series of alpha-pyronederivatives against Leishmania (L.) infantum and Trypanosoma cruzi. European Journal of Medicinal Chemistry, 2017, 139, 947-960. DOI: https://doi.org/10.1016/j.ejmech.2017.08.055
80 Muntoni, F.; Maresh, K.; Davies, K.; Harriman, S.; Layton, G.; Rosskamp, R.; Russell, A.; Tejura, B.; Tinsley, J. PhaseOut DMD: a Phase 2, proof of concept, clinical study of utrophin modulation with ezutromid. Neuromuscular Dis. 2017, 27, S217. DOI: https://doi.org/10.1016/j.nmd.2017.06.443.
79 Davies, S.G., Kennewell, P.D., Russell, A.J., Silpa, L., Westwood, R., and Wynne, G.M. Regenerative Medicine. Comprehensive Medicinal Chemistry III, Chackalamannil, S., Rotella, D., and Ward, S.E. (Ed.). Elsevier Ltd, 2017, ISBN: 978-0-12-803201-5.
78 Bataille, CJR.; Brennan, MB.; Byrne, S.; Davies, SG.; Durbin, M.; Fedorov, O.; Huber, KVM,; Jones, AM.; Knapp, S.; Liu, G.; Nadali, A.; Quevedoa, CE.; Russell, AJ.; Walker, RG.; Westwood, R.; Wynne, GW. Thiazolidine derivatives as potent and selective inhibitors of the PIM kinase family. Bioorganic and Medicinal Chemistry, 2017. DOI: http://dx.doi.org/10.1016/j.bmc.2017.02.056
2016
77 Zervou, S.; Whittington, H.J.; Russell, A.J. and Lygate, C.A. Augmentation of creatine in the heart. Mini-reviews in Medicinal Chemistry. 2016, 16, 19-28. DOI: 10.2174/1389557515666150722102151
76 Russell, A.J. and Silpa, L. Chemical-Induced Naive Pluripotency. Cell Chemical Biology, 2016, 23, 532-534. DOI: 10.1016/j.chembiol.2016.05.002.
75 McIntyre, A.; Hulikova, A.; Ledaki, I.; Snell, C.; Singleton, D.; Steers, G.; Seden, P.; Jones, D.; Bridges, E.; Winfield, S.; Li, J.L.; Russell, A.; Switch, P.; Harris, A.L. Disrupting Hypoxia-Induced Bicarbonate Transport Acidifies Tumor Cells and Suppresses Tumor Growth. Cancer Res, 2016, 76, 3744–55. DOI: 10.1158/0008-5472.CAN-15-1862
74 Davies, S. G.; Goddard, E, C.; Roberts, P. M.; Russell, A. J.; Smith, A. D.; Thomson, J. E.; Withey, J. M. Strategies for the construction of morphinan alkaloid AB-rings: regioselective Friedel-Crafts-type cyclisations of γ-aryl-β-benzoylamido acids with asymmetrically substituted γ-aryl rings. Tetrahedron: Asymmetry, 2016, 27, 274. DOI: https://doi.org/10.1016/j.tetasy.2016.02.010
2015
73 Davies, S. G.; Goddard, E. C.; Roberts, P. M.; Russell, A. J.; Thomson, J. E. Enantiopure 3-amino substituted 1-indanones, 1-tetralones and 1-benzosuberones via Friedel-Crafts cyclisation of ω-aryl-β-benzamido acids. Synlett, 2015. DOI: 10.1055/s-0034-1380675.
72 Brennan, M. B.; Davies, S. G.; Fletcher, A. M.; Lee, J. A.; Roberts, P. M.; Russell, A. J.; Thomson, J. E. Epoxidation of trans-4-aminocyclohex-2-en-1-ol derivatives: competition of hydroxyl-directed and ammonium-directed pathways. Aust. J. Chem. 2015, 68, 610-621. DOI: https://doi.org/10.1071/CH14531
71 Gianella-Borradori, M.; Christou, I.; Bataille, C. J. R.; Cross, R. L.; Wynne, G. M.; Greaves, D. R.; Russell, A. J. Ligand-based virtual screening identifies a family of selective cannabinoid receptor 2 agonists. Bioorg. Med. Chem. 2015, 23, 241-263. DOI: https://doi.org/10.1016/j.bmc.2014.11.002
70 Taylor, L.; Christou, I.; Kapellos, T. S.; Buchan, A.; Brodermann, M. H.; Gianella-Borradori, M.; Russell, A.; Iqbal, A. J.; Greaves, D. R. Primary macrophage chemotaxis induced by cannabinoid receptor 2 agonists occurs independently of the CB2 receptor. Scientific Rep. 2015, 5, 10682. DOI: 10.1038/srep10682
69 Guiraud, S.; Squire, S. E.; Edwards, B.; Chen, H.; Burns, D. T.; Shah, N.; Babbs, A.; Davies, S. G.; Wynne, G. M.; Russell, A. J.; Elsey, D.; Wilson, F. X.; Tinsley, J. M.; Davies, K. E. Second-generation compound for the modulation of utrophin in the therapy of DMD. Hum. Mol. Genet. 2015, DOI: 10.1093/hmg/ddv154.
68 Davies, S. G.; Kennewell, P. D.; Russell, A. J.; Seden, P. T.; Westwood, R.; Wynne, G. M. Stemistry: The Control of Stem Cells in Situ Using Chemistry. J. Med. Chem. 2015, 58, 2863-2894. DOI: 10.1021/jm500838d
67 Brennan, M.; Csatayová, K.; Davies, S. G.; Fletcher, A. M.; Green, W. D.; Lee, J. A.; Roberts, P. M.; Russell, A. J.; Thomson, J. E. Syntheses of dihydroconduramines (±)-B-1, (±)-E-1 and (±)-F-1 via diastereoselective epoxidation of N-protected 4-aminocyclohex-2-en-1-ols. J. Org. Chem. 2015, DOI: 10.1021/acs.joc.5b00716.
66 Clunie-O’Connor, C.; Smits, A.M.; Antoniades, C.; Russell, A.J.; Yellon, D.M.; Goumans, M-J.; Riley, P.R. The Derivation of Primary Human Epicardium-Derived Cells. Curren. Protoc. Stem Cell Biol. 2015, 35: 2C.5.1 - 2C.5.x. DOI: 10.1002/9780470151808.sc02c05s35
2014
65 Laurieri, N.; Kawamura, A.; Westwood, I. M.; Varney, A.; Morris, E.; Russell, A. J.; Stanley, L. A.; Sim, E. Differences between murine arylamine N-acetyltransferase type 1 and human arylamine N-acetyltransferase type 2 defined by substrate specificity and inhibitor binding. BMC Pharmacol. Toxicol. 2014, 15, 68. DOI: 10.1186/2050-6511-15-68
64 Russell, A. J. and Wynne, G. M.
Drug Discovery Approaches for Rare Neuromuscular Diseases. Orphan Drugs and Rare Diseases, Palmer, M. and Pryde, D. (Ed.). Royal Society of Chemistry, 2014, ISBN: 978-1-84973-806-4.
63 Abuhammad, A.; Fullam, E.; Bhakta, S.; Russell, A. J.; Morris, G. M.; Finn, P. W.; Sim, E. Exploration of Piperidinols as Potential Antitubercular Agents. Molecules, 2014, 19, 16274–16290. DOI: 10.3390/molecules191016274
62 Laurieri, N.; Dairou, J.; Egleton, J. E.; Stanley, L. A.; Russell, A. J.; Dupret, J. M.; Sim, E.; Rodrigues-Lima, F. From Arylamine N-Acetyltransferase to Folate-Dependent Acetyl CoA Hydrolase: Impact of Folic Acid on the Activity of (HUMAN)NAT1 and Its Homologue (MOUSE)NAT2. PLoS One, 2014, 9, e96370. DOI: 10.1371/journal.pone.0096370
61 Egleton, J. E.; Thinnes, C. C.; Seden, P. T.; Laurieri, N.; Lee, S. P.; Hadavizadeh, K. S.; Measures, A. R.; Jones, A. M.; Thompson, S.; Varney, A.; Wynne, G. M.; Ryan, A.; Sim, E.; Russell, A. J. Structure-activity relationships and colorimetric properties of specific probes for the putative cancer biomarker human arylamine N-acetyltransferase 1. Bioorg. Med. Chem. 2014, 22, 3030–3054. DOI: 10.1016/j.bmc.2014.03.015
60 Davies, S. G. and Russell, A. J.
Chemical Biology of Stem Cell Modulation
New Frontiers in Chemical Biology, Bunnage, M. E. (Ed.). Royal Society of Chemistry, 2011, ISBN: 184973125X.
2013
59 Lufino, M. M. P.; Silva, A. M.; Németh, A. H.; Alegre-Abarrategui J.; Russell A. J.; Wade-Martins, R. A. A GAA repeat expansion reporter model of Friedreich's ataxia recapitulates the genomic context and allows rapid screening of therapeutic compounds. Human Mol. Genet. 2013, 22, 5173–5187. DOI: 10.1093/hmg/ddt370
58 Laurieri, N.; Egleton, J.E.; Varney, A.; Thinnes, C. C.; Quevedo, C. E.; Seden, P.T.; Thompson, S.; Rodrigues-Lima, F.; Dairou, J.; Dupret, J.-M.; Russell, A. J.; Sim, E. A Novel Color Change Mechanism for Breast Cancer Biomarker Detection: Naphthoquinones as Specific Ligands of Human Arylamine N-Acetyltransferase 1. PLoS One, 2013, 8, e70600. DOI: 10.1371/journal.pone.0070600
57 Fullam, E.; Talbot, J.; Abuhammed, A.; Westwood, I.; Davies, S. G.; Russell, A. J.; Sim, E. Design, synthesis and structure-activity relationships of 3,5-diaryl-1H-pyrazoles as inhibitors of arylamine N-acetyltransferase. Bioorg. Med. Chem. Lett. 2013, 23, 2759–2764. DOI: 10.1016/j.bmcl.2013.02.052
56 Hewings, D. S.; Fedorov, O.; Filippakopoulos, P.; Martin, S.; Picaud, S.; Tumber, A.; Wells, C.; Olcina, M. M.); Freeman, K.; Gill, A.; Ritchie, A. J.; Sheppard, D. W.; Russell, A. J.; Hammond, E. M.; Knapp, S.; Brennan, P. E.; Conway, S. J. Optimization of 3,5-Dimethylisoxazole Derivatives as Potent Bromodomain Ligands. J. Med. Chem. 2013, 56, 3217–3227. DOI: 10.1021/jm301588r
55 Russell, A. J. Regenerative Medicinal Chemistry: The in Situ Control of Stem Cells. ACS Med. Chem. Lett. 2013, 4, 365–368. DOI: 10.1021/ml400110b
54 Claridge, T. D. W.; Davies, S. G.; Kruchinin, D.; Odell, B.; Roberts, P. M.; Russell, A. J.; Thomson, J. E.; Toms, S. M. Solution phase structures of enantiopure and racemic lithium N-benzyl-N-(α-methylbenzyl)amide in THF: Low temperature Li and N NMR spectroscopic studies. Tetrahedron Asymmetry, 2013, 24, 947–952. DOI: https://doi.org/10.1016/j.tetasy.2013.07.001
2012
53 Abuhammad, A.; Fullam, E.; Lowe, E. D.; Staunton, D.; Kawamura, A.; Westwood, I. M.; Bhakta, S.; Garner, A. C.; Wilson, D. L.; Seden, P. T.; Davies, S. G.; Russell, A. J.; Garman, E. F.; Sim, E. Piperidinols That Show Anti-Tubercular Activity as Inhibitors of Arylamine N-Acetyltransferase: An Essential Enzyme for Mycobacterial Survival Inside Macrophages. PLoS One, 2012, 7, e52790. DOI: 10.1371/journal.pone.0052790
52 Davies, S. G.; Fletcher, A. M.; Lee, J. A.; Roberts, P. M.; Russell, A. J.; Taylor, R. J.; Thomson, A. D.; Thomson, J. E. Polysubstituted Piperidines via Iodolactonization: Application to the Asymmetric Synthesis of (+)-Pseudodistomin D. Org. Lett. 2012, 14, 1672–1675. DOI: 0.1021/ol300209s
2011
51 Abraham, E.; Claridge, T. D. W.; Davies, S. G.; Odell, B.; Roberts, P. M.; Russell, A. J.; Smith, A. D.; Smith, L. J.; Storr, H. R.; Sweet, M. J.; Thompson, A. L.; Thomson, J. E.; Tranter, G. E.; Watkin, D. J. A systematic study of the solid state and solution phase conformational preferences of beta-peptides derived from C(3)-alkyl substituted transpentacin derivatives. Tetrahedron: Asymmetry, 2011, 22, 69–100. DOI: https://doi.org/10.1016/j.tetasy.2010.12.007
50 Fullam, E.; Abuhammad, A.; Wilson, D. L.; Davies, S. G.; Russell, A. J. and Sim, E. Analysis of beta-amino alcohols as inhibitors of the potential anti-tubercular target N-acetyltransferase. Bioorg. Med. Chem. Lett. 2011, 21, 1185–1190. DOI: 10.1016/j.bmcl.2010.12.099
49 Davies, S. G.; Fletcher, A. M.; Hughes, D. G.; Lee, J. A.; Price, P. D.; Roberts, P. M.; Russell, A. J.; Smith, A. D.; Thomson, J. E.; Williams, O. M. H. Asymmetric synthesis of piperidines and octahydroindolizines using a one-pot ring-closure/N-debenzylation procedure. Tetrahedron 2011, 67, 9975–9992. DOI: https://doi.org/10.1016/j.tet.2011.09.038
48 Li, J. L.; Sainson, R. C. A.; Oon, C. E.; Turley, H.; Leek, R.; Sheldon, H.; Bridges, E.; Shi, W.; Snell, C.; Bowden, E. T.; Wu, H. R.; Chowdhury, P. S.; Russell, A. J.; Montgomery, C. P.; Poulsom, R. and Harris, A. L. DLL4-Notch Signaling Mediates Tumor Resistance to Anti-VEGF Therapy In Vivo. Cancer Res. 2011, 71, 6073–6083. DOI: 10.1158/0008-5472.CAN-11-1704
47 Csatayova, K.; Davies, S. G.; Lee, J. A.; Roberts, P. M.; Russell, A. J.; Thomson, J. E.; Wilson, D. L. Highly Diastereoselective and Stereodivergent Dihydroxylations of Acyclic Allylic Amines: Application to the Asymmetric Synthesis of 3,6-Dideoxy-3-amino-L-talose. Org. Lett. 2011, 13, 2606–2609. DOI: 10.1021/ol200717n
46 Westwood, I. M.; Kawamura, A.; Russell, A. J.; Sandy, J.; Davies S. G. and Sim, E. Novel Small-Molecule Inhibitors of Arylamine N-Acetyltransferases: Drug Discovery by High Throughput Screening. Combi. Chem. High Throughput Screening 2011, 14, 117–124. DOI: 10.2174/138620711794474051
45 Davies, S. G. and Russell, A. J.
Chemical Biology of Stem Cell Modulation
New Frontiers in Chemical Biology, Bunnage, M. E. (Ed.). Royal Society of Chemistry, 2011, ISBN: 184973125X.
2010
44 Abraham, E.; Bailey, C. W.; Claridge, T. D. W.; Davies, S. G.; Ling, K. B.; Odell, B.; Rees, T. L.; Roberts, P. M.; Russell, A. J.; Smith, A. D.; Smith, L. J.; Storr, H. R.; Sweet, M. J.; Thompson, A. L.; Thomson, J. E.; Tranter, G. E.; Watkin, D. J. A systematic study of the solid state and solution phase conformational preferences of beta-peptides derived from transpentacin. Tetrahedron: Asymmetry 2010, 21, 1797–1815. DOI: https://doi.org/10.1016/j.tetasy.2010.12.007
43 Bagal, S. K.; Davies, S. G.; Lee, J. A.; Roberts, P. M.; Russell, A. J.; Scott, P. M. and Thomson, J. E. An Oxidation and Ring Contraction Approach to the Synthesis of (+/-)-1-Deoxynojirimycin and (+/-)-1-Deoxyaltronojirimycin. Org. Lett. 2010, 12, 136–139. DOI: 10.1021/ol902533b
42 Davies, S. G.; Hughes, D. G.; Price, P. D.; Roberts, P. M.; Russell, A. J.; Smith, A. D.; Thomson, J. E. And Williams, O. M. H. Asymmetric Synthesis of Piperidines and Octahydroindolizines. Synlett 2010, 567–570. DOI: https://doi.org/10.1016/j.tet.2011.09.038
41 Csatayova, K.; Davies, S. G.; Lee, J. A.; Ling, K. B.; Roberts, P. M.; Russell, A. J.; Thomson, J. E. Chemo- and diastereoselective cyclopropanation of allylic amines and carbamates. Tetrahedron 2010, 66, 8420–8440. DOI: https://doi.org/10.1016/j.tet.2010.08.055
40 Bentley, S. A.; Davies, S. G.; Lee, J. A.; Roberts, P. M.; Russell, A. J.; Thomson, J. E.; Toms, S. M. Conjugate addition of lithium N-tert-butyldimethylsilyloxy-N-(alpha-methylbenzyl)amide: asymmetric synthesis of beta(2,2,3)-trisubstituted amino acids. Tetrahedron, 2010, 66, 4604–4620. DOI: https://doi.org/10.1016/j.tet.2010.04.027
39 Westwood, I. M.; Bhakta, S.; Russell, A. J.; Fullam, E.; Anderton, M. C.; Kawamura, A.; Mulvaney, A. W.; Vickers, R. J.; Bhowruth, V.; Besra, G. S.; Lalvani, A.; Davies, S. G. and Sim, E. Identification of arylamine N-acetyltransferase inhibitors as an approach towards novel anti-tuberculars. Protein & Cell, 2010, 1, 82-95. DOI: 10.1007/s13238-010-0006-1
38 Laurieri, N.; Crawford, M. H. J.; Kawamura, A.; Westwood, I. M.; Robinson, J.; Fletcher, A. M.; Davies, S. G.; Sim, E. and Russell, A. J. Small Molecule Colorimetric Probes for Specific Detection of Human Arylamine N-Acetyltransferase 1, a Potential Breast Cancer Biomarker. J. Am. Chem. Soc. 2010, 132, 3238–3239. DOI: 10.1021/ja909165u
37 Csatayova, K.; Davies, S. G.; Lee, J. A.; Ling, K. B.; Roberts, P. M.; Russell, A. J.; Thomson, J. E. Syntheses of trans-SCH-A and cis-SCH-A via a Stereodivergent Cyclopropanation Protocol. Org. Lett. 2010, 12, 3152–3155. DOI: 10.1021/ol101295t
36 Davies, S. G.; Ling, K. B.; Roberts, P. M.; Russell, A. J.; Thomson, J. E.; Woods, P. A. The stereodivergent aziridination of allylic carbamates, amides and sulphonamides. Tetrahedron 2010, 66, 6806–6813. DOI: https://doi.org/10.1016/j.tet.2010.06.059
35 Cresswell, A. J.; Davies, S. G.; Lee, J. A.; Roberts, P. M.; Russell, A. J.; Thomson, J. E.; Tyte, M. J. beta-Fluoroamphetamines via the Stereoselective Synthesis of Benzylic Fluorides. Org. Lett. 2010, 12, 2936–2939. DOI: 10.1021/ol100862s
2009
34 Pettigrew, D. M.; Roversi, P.; Davies, S. G; Russell, A. J. and Lea, S. M. A structural study of the interaction between the Dr haemagglutinin DraE and derivatives of chloramphenicol. Acta Cryst. D 2009, 65, 513–522. DOI: 10.1107/S0907444909005113
33 Soncin, F.; Mohamet, L.; Eckardt, D.; Ritson, S.; Eastham, A. M.; Spencer, H.; Bobola, N.; Russell, A. J.; Davies, S. G.; Kemler, R.; Merry, C. L. R. and Ward, C. M. Abrogation of E-cadherin mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells, 2009, 27, 2069–2080. DOI: 10.1002/stem.134
32 Bond, C. W.; Cresswell, A. J.; Davies, S. G.; Fletcher, A. M.; Kurosawa, W.; Lee, J. A.; Roberts, P. M.; Russell, A. J.; Smith, A. D. and Thomson, J. E. Ammonium-Directed Oxidation of Cyclic Allylic and Homoallylic Amines. J. Org. Chem. 2009, 74, 6735–6748. DOI: 10.1021/jo9012783
31 Torabi Kachoosangi, R.; Musameh, M. M.; Abu-Yousef, I.; Yousef, J. M.; Kanan, S. M.; Xiao, L.; Davies, S. G.; Russell, A. and Compton, R. G. Carbon Nanotube - Ionic Liquid Composite Sensors and Biosensors. Anal. Chem. 2009, 81, 435–442. DOI: 10.1021/ac801853r
30 Davies, S. G.; Durbin, M. J; Goddard, E. C.; Kelly, P. M.; Kurosawa, W.; Lee, J. A.; Nicholson, R. L.; Price, P. D.; Roberts, P. M.; Russell, A. J.; Scott, P. M. and Smith, A. D. Double diastereoselective conjugate addition of homochiral lithium amides to homochiral α,β-unsaturated esters containing cis- and trans-dioxolane units. Org. Biomol. Chem. 2009, 7, 761–776. DOI: 10.1039/B818298A
29 Wilkinson, R. N.; Gering, M.; Russell, A. J.; Davies, S. G.; Kimelman, D. and Patient, R. Hedgehog and Bmp Polarize Hematopoietic Stem Cell Emergence in the Zebrafish Dorsal Aorta. Dev. Cell. 2009, 16, 909–916. DOI: 10.1016/j.devcel.2009.04.014
28 Aciro, C.; Davies, S. G.; Kurosawa, W.; Roberts, P. M.; Russell, A. J. and Thomson, J. E. Highly diastereoselective anti-dihydroxylation of 3-N,N-dibenzylaminocyclohex-1-ene N-oxide. Org. Lett. 2009, 11, 1333–1336. DOI: 10.1021/ol900114b
27 Davies, S. G.; Nicholson, R. L.; Price, P. D.; Roberts, P. M.; Russell, A. J.; Savory, E. D.; Smith, A. D. and Thomson, J. E. Iodine-mediated ring-closing iodoamination with concomitant N-debenzylation for the asymmetric synthesis of polyhydroxylated pyrrolidines. Tetrahedron: Asymmetry, 2009, 20, 758–772. DOI: https://doi.org/10.1016/j.tetasy.2009.02.014
26 Russell, A. J.; Westwood, I. M.; Crawford, M. C.; Robinson, J.; Kawamura, A.; Redfield, C.; Lowe, E. D.; Laurieri, N.; Davies, S. G. and Sim, E. Selective small molecule inhibitors of the potential breast cancer marker, human arylamine N-acetyltransferase 1, and its murine homologue, mouse arylamine N-acetyltransferase 2. Bioorg. Med. Chem. 2009, 17, 905–918. DOI: 10.1016/j.bmc.2008.11.032
25 Lack, N. A.; Kawamura, A.; Fullam, E.; Laurieri, N.; Beard, S.; Russell, A. J.; Evangelopoulos, D.; Westwood I. and Sim, E. Temperature stability of proteins essential for the intracellular survival of Mycobacterium tuberculosis. Biochem. J. 2009, 418, 369–378. DOI: 10.1042/BJ20082011
2008
24 Claridge, T. D. W.; Davies, S. G.; Polywka, M. E. C.; Roberts, P. M.; Russell, A. J.; Savory, E. D. and Smith, A. D. “Pure by NMR?” Org. Lett. 2008, 10, 5433–5436. DOI: 10.1021/ol802211p
23 Aciro, C.; Claridge, T. D. W.; Davies, S. G.; Roberts, P. M.; Russell, A. J. and Thomson, J. E. Ammonium directed dihydroxylation of 3-amino-cyclohex-1-enes: development of a metal-free dihydroxylation protocol. Org. Biomol. Chem. 2008, 20, 3751–3761. DOI: 10.1039/B808811J
22 Aciro, C.; Davies, S. G.; Roberts, P. M.; Russell, A. J.; Smith, A. D. and Thomson, J. E. Ammonium directed dihydroxylation: metal-free synthesis of the diastereoisomers of 3-amino-cyclohexane-1,2-diol. Org. Biomol. Chem. 2008, 20, 3762–3770. DOI: 10.1039/B808812H
21 Davies, S. G.; Mortimer, D. A. B.; Mulvaney, A. W.; Russell, A. J.; Skarphedinsson, H.; Smith A. D. and Vickers, R. J. An oxidatively-activated safety catch linker for solid phase synthesis. Org. Biomol. Chem., 2008, 6, 1625–1634. DOI: 10.1039/B802204F
20 Abraham, E.; Brock, E. A.; Candela-Lena, J. I.; Davies, S. G.; Georgiou, M.; Nicholson, R. L.; Perkins, J. H.; Roberts, P. M.; Russell, A. J.; Sánchez-Fernández, E. M.; Scott, P. M.; Smith, A. D. and Thomson, J. E. Asymmetric synthesis of N,O,O,O-tetra-acetyl D-lyxo-phytosphingosine, jaspine B (pachastrissamine), 2-epi-jaspine B, and deoxoprosophylline via lithium amide conjugate addition. Org. Biomol. Chem., 2008, 6, 1665–1673. DOI: 10.1039/B801671B
19 Abraham, E.; Candela-Lena, J. I.; Davies, S. G.; Georgiou, M.; Nicholson, R. L.; Roberts, P. M.; Russell, A. J.; Sanchez-Fernandez, E. M.; Smith, A. D.; Thomson, J. E. Asymmetric synthesis of N,O,O,O-tetra-acetyl D-lyxo-phytosphingosine, jaspine B (pachastrissamine) and its C(2)-epimer. Tetrahedron: Asymmetry 2008, 18, 2510–2513. DOI: https://doi.org/10.1016/j.tetasy.2007.10.026
18 Case-Green, S. C.; Davies, S. G.; Roberts, P. M.; Russell, A. J. and Thomson, J. A. Asymmetric synthesis of tetrahydrolipstatin and valilactone. Tetrahedron: Asymmetry, 2009, 19, 2870–2881. DOI: https://doi.org/10.1016/j.tetasy.2008.11.012
17 Claridge, T. D. W.; Davies, S. G.; Lee, J. A.; Nicholson, R. L.; Roberts, P. M.; Russell, A. J.; Smith, A. D. and Toms, S. M. Highly (E)-Selective Wadsworth-Emmons Reactions Promoted by Methylmagnesium Bromide. Org. Lett. 2008, 10, 5437–5440. DOI: 10.1021/ol802212e
16 Musameh, M. M.; Kachoosangi, R. T.; Xiao, L.; Russell, A. and Compton, R. G. Ionic liquid-carbon composite glucose biosensor. Biosensors and Bioelectronics, 2008, 24, 87–92. DOI: https://doi.org/10.1016/j.bios.2008.03.015
15 Abraham, E.; Davies, S. G.; Roberts, P. M.; Russell, A. J. and Thomson, J. E. Jaspine B (pachastrissamine) and 2-epi-jaspine B: synthesis and structural assignment. Tetrahedron: Asymmetry, 2008, 19, 1027–1047. DOI: https://doi.org/10.1016/j.tetasy.2008.04.017
14 Abraham, E.; Davies, S. G.; Docherty, A. J.; Ling, K. B.; Roberts, P. M.; Russell, A. J.; Thomson, J. E. and Toms, S. M. Parallel kinetic resolution of methyl (RS)-5-tris(phenylthio)methyl-cyclopent-1-ene-carboxylate for the asymmetric synthesis of (1R,2S,5S)- and (1S,2R,5R)-5-methyl-cispentacin. Tetrahedron: Asymmetry, 2008, 19, 1356–1362. DOI: https://doi.org/10.1016/j.tetasy.2008.05.016
13 Davies, S. G.; Durbin, M. J.; Hartman, S. J. S.; Matsuno, A.; Roberts, P. M.; Russell, A. J.; Smith, A. D.; Thomson, J. E. and Toms, S. M. Parallel kinetic resolution of tert-butyl (RS)-6-alkyl-cyclohex-1-ene-carboxylates for the asymmetric synthesis of 6-alkyl-substituted cishexacin derivatives. Tetrahedron: Asymmetry 2009, 19, 2870–2881. DOI: https://doi.org/10.1016/j.tetasy.2008.11.019
12 Aciro, C.; Davies, S. G.; Garner, A. C.; Ishii, Y.; Key, M. S.; Ling, K. B.; Prasad, R. S.; Roberts, P. M.; Rodriguez-Solla, H.; O’Leary-Steele, C.; Russell, A. J.; Sanganee, H. J.; Savory, E. D.; Smith, A. D. and Thomson, J. E. Stereoselective functionalisation of SuperQuat enamides: asymmetric synthesis of homochiral 1,2-diols and α-benzyloxy carbonyl compounds. Tetrahedron, 2008, 64, 9320–9344. DOI: https://doi.org/10.1016/j.tet.2008.07.012
2007
11 Cailleau, T.; Cooke, J. W. B.; Davies, S. G.; Ling, K. B.; Naylor, A.; Nicholson, R. L.; Price, P. D.; Roberts, P. M.; Russell, A. J.; Smith, A. D.; Thomson, J. E. Asymmetric synthesis of beta-amino-gamma-substituted-gamma-butyrolactones: double diastereoselective conjugate addition of homochiral lithium amides to homochiral alpha,beta-unsaturated esters. Org. Biomol. Chem. 2007, 5, 3922–3931. DOI: 10.1039/B712937H
10 Beddow, J. E.; Davies, S. G.; Ling, K. B.; Roberts, P. M.; Russell, A. J.; Smith, A. D.; Thomson, J. E. Asymmetric synthesis of beta(2)-amino acids: 2-substituted-3-aminopropanoic acids from N-acryloyl SuperQuat derivatives. Org. Biomol. Chem. 2007, 5, 2812–2825. DOI: 10.1039/B707689D
9 Davies, S. G.; Ling, K. B.; Roberts, P. M.; Russell, A. J.; Thomson, J. E. Diastereoselective Simmons-Smith cyclopropanations of allylic amines and carbamates. Chem. Commun. 2007, 39, 4029–4031. DOI: 10.1039/B711358G
8 Rogers, E. I.; Silvester, D. S.; Jones, S. E. W.; Aldous, L.; Hardacre, C.; Russell, A. J.; Davies, S. G.; Compton, R. G. Electrochemical kinetics of AgAg+ and TMPDTMPD+ in the room-temperature ionic liquid [C(4)mpyrr][NTf2]; toward optimizing reference electrodes for voltammetry in RTILs. J. Phys. Chem. C 2007, 111, 13957–13966. DOI: 10.1021/jp0737754
7 Davies, S. G.; Russell, A. J.; Sheppard, R. L.; Smith, A. D.; Thomson, J. E. Evaluating beta-amino acids as enantioselective organocatalysts of the Hajos-Parrish-Eder-Sauer-Wiechert reaction. Org. Biomol. Chem. 2007, 5, 3190–3200. DOI: 10.1039/B711171A
6 Madikane, V. E.; Bhakta, S.; Russell, A. J.; Campbell, W. E.; Claridge, T. D. W.; Elisha, B. G.; Davies, S. G.; Smith, P.; Sim, E. Inhibition of mycobacterial arylamine N-acetyltransferase contributes to anti-mycobacterial activity of Warburgia salutaris. Bioorg. Med. Chem. 2007, 15, 3579–3586. DOI: https://doi.org/10.1016/j.bmc.2007.02.011
5 Davies, S. G.; Mulvaney, A. W.; Russell, A. J.; Smith, A. D. Parallel synthesis of homochiral beta-amino acids. Tetrahedron: Asymmetry, 2007, 18, 1554–1566. DOI: https://doi.org/10.1016/j.tetasy.2007.06.008
pre-2007
4 Candela-Lena, J. I.; Davies, S. G.; Roberts, P. M.; Roux, B.; Russell, A. J.; Sanchez-Fernandez, E. M.; Smith, A. D. Asymmetric synthesis of alpha-mercapto-beta-amino acid derivatives: application to the synthesis of polysubstituted thiomorpholines. Tetrahedron: Asymmetry 2006, 17, 1135–1145. DOI: 10.1016/j.tetasy.2006.04.004
3 Davies, S. G.; Garrido, N. M.; Kruchinin, D.; Ichihara, O.; Kotchie, L. J.; Price, P. D.; Mortimer, A. J. P.; Russell, A. J.; Smith, A. D. Homochiral lithium amides for the asymmetric synthesis of beta-amino acids. Tetrahedron: Asymmetry, 2006, 17, 1793–1811. DOI: https://doi.org/10.1016/j.tetasy.2006.05.008
2 Westwood, I. M.; Kawamura, A.; Fullam, E.; Russell, A. J.; Davies, S. G.; Sim, E. Structure and mechanism of arylamine N-acetyltransferases. Curr. Top. Med. Chem. 2006, 6, 1641–1654. DOI: 10.2174/156802606778108979
1 Beddow, J. E.; Davies, S. G.; Smith, A. D.; Russell, A. J. Asymmetric synthesis of 2-alkyl- and 2-aryl-3-aminopropionic acids (beta(2)-amino acids) from (S)-N-acryloyl-5,5-dimethyloxazolidin-2-one SuperQuat derivatives. Chem. Commun. 2004, 23, 2778–2779. DOI: 10.1039/B410938D