Regenerative medicine
Stem cell chemistry - "Stemistry"
The ability to harness the potential of stem and precursor cells would be a major advance in the treatment of human disease. Stem cells are remarkable cells characterised by their ability to divide and to differentiate via a number of steps to embryonic and adult somatic cell lineages. Such cells thus hold enormous promise both for in vitro screening tools for drug efficacy and toxicity testing, and for regenerative therapies treating a wide range of disorders with high unmet medical need such as neurodegenerative diseases, diabetes, heart disease, and vision loss.
The ability to control each step in proliferation, differentiation and redifferentiation/reprogramming processes would allow control of the selective production of different tissue types both in vitro and, importantly in many instances, directly in vivo. The use of chemicals to manipulate cell fate offers many significant advantages over other techniques in terms of speed, cost, reproducibility and the ability to influence cell fate reversibly. To this end through a number of collaborative programmes we have discovered and developed small molecules to selectively manipulate cell fate in vitro and in vivo, for which we have coined the term “stemistry” (stem cell chemistry) (1, 2, 3).
References
(1) Davies, S. G.; Kennewell, P. D.; Russell, A. J.; Seden, P. T.; Westwood, R.; Wynne, G. M. Stemistry: The Control of Stem Cells in Situ Using Chemistry. J. Med. Chem. 2015, 58, 2863-2894.
(2) Russell, A. J. Regenerative Medicinal Chemistry: The in Situ Control of Stem Cells. ACS Med. Chem. Lett. 2013, 4, 365–368.
(3) Wilkinson, R. N.; Gering, M.; Russell, A. J.; Davies, S. G.; Kimelman, D. and Patient, R. Hedgehog and Bmp Polarize Hematopoietic Stem Cell Emergence in the Zebrafish Dorsal Aorta. Dev. Cell. 2009, 16, 909–916.
Identification of small molecule modulators to enable bulk production of stem and/or progenitor cells in 3D single cell suspension culture
With Professor Sally Cowley and Professor William James, Sir William Dunn School of Pathology, University of Oxford

Induced pluripotent stem cells (iPSCs), which can be derived from mature differentiated cells, provide an excellent source of cells to study human diseases mechanisms, develop regenerative therapies and screen for lead molecules for drug discovery. A fundamental element needed to fully exploit the potential of stem cells throughout these applications is the ability to produce a large number of cells of a consistent quality and in a cost effective manner.
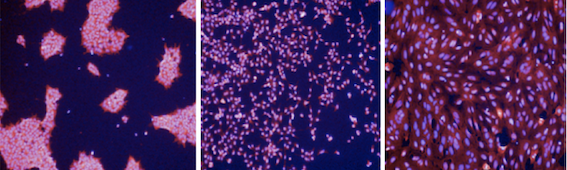
hiPSCs grown in the absence (left panel) and presence (centre and right panels) of modulators of cell-cell adhesion
In previous work, we identified compounds which reversibly blocked differentiation and cell-cell contact in mouse embryonic stem cells, based on small molecule mimics of key structural motifs within the cell surface adhesion protein E-cadherin.
A combination of molecular modelling, ligand based-design and focused library screening is currently being employed to develop small molecules to allow for the first time the scaleable 3D suspension culture of human induced pluripotent stem cells (iPSCs). These chemicals will form a platform of crucial medium additives to facilitate the large-scale growth of stem cells in 3D suspension culture, an immensely valuable experimental tool with potential to deliver a huge impact in developing healthcare technologies.
References
(1) Soncin, F.; Mohamet, L.; Eckardt, D.; Ritson, S.; Eastham, A. M.; Spencer, H.; Bobola, N.; Russell, A. J.; Davies, S. G.; Kemler, R.; Merry, C. L. R. and Ward, C. M. Abrogation of E-cadherin mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells, 2009, 27, 2069–2080.
Links
Small molecule activation of the epicardium for cardiac repair
With Professor Paul Riley (Dept of Physiology, Anatomy and Genetics), Professor Roger Patient (Weatherall Institute of Molecular Medicine; WIMM), and Professor Robin Choudhury (Radcliffe Department of Medicine)


The inability of the human heart to functionally repair itself in response to injury remains the biggest cause of morbidity and mortality in the global population. The most catastrophic injury, a myocardial infarction (MI), results in the instantaneous death of millions of cardiomyocytes that cannot be sufficiently regenerated by endogenous repair mechanisms. A new strategy to activate cardiac regeneration is the use of small molecules acting directly on resident cardiac progenitors, stimulating proliferation, migration, differentiation and maturation.
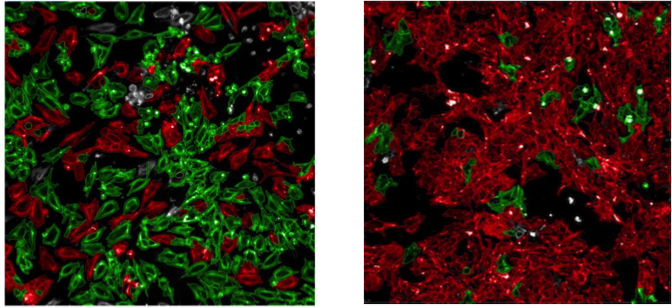
Human epicardial cells in the presence (left panel) or absence (right panel) of an activator of epithelial-to-mesenchymal transition.
Our collaborators have previously shown that epicardial cells can differentiate into cardiomyocytes, fibroblasts and vasculature. Furthermore, upon treatment with peptide thymosin β4, endogenous epicardial cells migrate and differentiate into cardiomyocytes in situ following injury (Smart et al. Nature 2011, 474, 640). However, this represents only a limited regenerative capacity. We have established screens both in vitro and in vivo to allow the discovery of small molecules that will augment the progenitor activation and replacement of destroyed myocardium. We have identified several examples of candidate molecules showing activity in vitro.
References
(1) Clunie-O’Connor, C.; Smits, A.M.; Antoniades, C.; Russell, A.J.; Yellon, D.M.; Goumans, M-J.; Riley, P.R. The Derivation of Primary Human Epicardium-Derived Cells. Curren. Protoc. Stem Cell Biol. 2016, 35: 2C.5.1 - 2C.5.x.
Links
Discovery of proneurogenic drug candidates: a new therapeutic strategy for neurodegenerative disorders
With Professor Francis Szele (Department of Physiology, Anatomy and Genetics) and Professor Noel Buckley (Department of Psychiatry), University of Oxford

Neurodegenerative diseases exert a vast physical, emotional and economic cost on patients and society, and with an ageing population their prevalence is rapidly increasing. There are currently estimated to be 47m people living with dementia globally, costing over $800b each year, but this is predicted to rise nearly three-fold by 2050 (World Alzheimer's Report 2015).
We aim to tackle neurodegenerative diseases by targeting the endogenous neural stem cells (NSCs) already present within the adult brain, stimulating natural repair mechanisms. This could be utilised as a novel treatment for a range of conditions such as Alzheimer’s disease, Parkinson’s disease, and traumatic brain injury. NSCs are found within two main neurogenic niches; the subgranular zone (SGZ) of the hippocampal dentate gyrus, and the subventricular zone (SVZ) of the lateral ventricles.
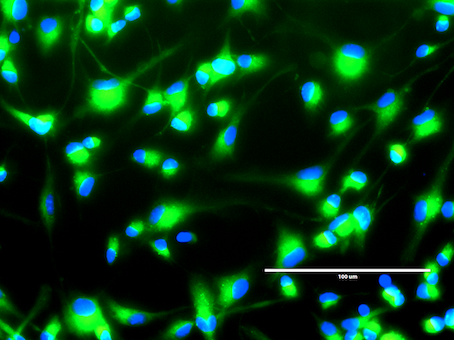
Fluorescent microscope image showing the localisation of a BODIPY-tagged compound derivative in SVZ-derived neural stem cells. (Blue: Hoechst nuclear stain; Green: BODIPY-tagged compound)
We have developed a semi-automated in vitro phenotypic assay, using a monolayer of primary murine NSCs (isolated from SGZ or SVZ) and measuring the appearance of mature neurons. We have used this assay to perform a pilot screen of 1500 compounds, from which we identified 30 compounds which induced a significant increases in neurogenesis. The use of a phenotypic assay gives us the opportunity to utilise a hypothesis-free and target agnostic approach, whilst also allowing a more direct translation of results into in vivo studies. Following preliminary pharmacokinetic evaluation, early in vivo efficacy work was conducted, wherein one lead compound was found to give a significant enhancement in SGZ neurogenesis after oral administration to wild-type mice. As a result, this compound has now been progressed to Alzheimer’s disease models.
Identification of small molecule agents that can stimulate precursor cells within the retina of patients for retinal repair and restoration of vision
With Professor Robert MacLaren, Nuffield Department of Clinical Neurosciences, University of Oxford

Retinal degeneration is the leading cause of untreatable blindness in the world. Age-related Macular Degeneration (AMD), for instance, affects approximately 14 million people, and is due to a loss of retinal pigment epithelial (RPE) cells and secondary photoreceptor death, leading to disabling central visual deficits in the elderly.
One strategy to ameliorate these diseases is to replace the lost retinal cell types in an effort to re-establish the circuitry for visual sensation.
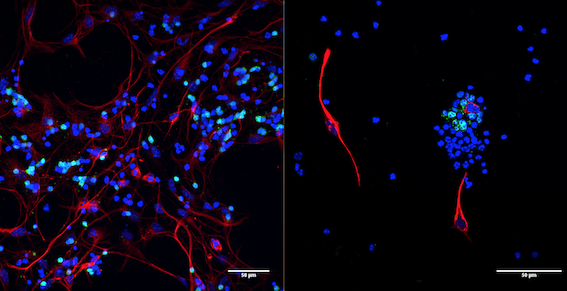
Primary cultures of retinal cells at post-natal day 7 (left panel) and post-natal day 13 (right panel)
In collaboration with Professor Robert MacLaren's group we are aiming to identify agents that will stimulate appropriate retinal precursor cells within the retina of patients with a range of retinopathies to stimulate retinal repair to restore vision.